eCoin Implantable Device to Control Urgent Urinary Incontinence
Peripheral neurostimulator for tibial nerve stimulation for incontinence treatment is delivered through an implantable device.
Late last year, the FDA granted a PMA to Valencia Technologies, a neuromodulation company, for its neurostimulator as a treatment for urgency urinary incontinence.1 The tibial nerve, which supplies electrical signals to muscles in the lower leg via the brain, can also play a role in bladder control. The tibial nerve is connected to nerves that innervate the bladder,2 an organ that for millions of women doesn’t work like it once did. Urinary incontinence, of all types, affects at least 60% of women in this country, as of 2018, according to government data. Reasons for the increase include an aging population and obesity.3
Tibial nerve stimulation is one incontinence treatment type, delivered with a needle electrode and a handheld stimulator. Patients visit their healthcare provider every week for 12 weeks, in a half-hour treatment.4 But a new treatment called the eCoin peripheral neurostimulator eliminates the weekly treatment requirements. Studies show the eCoin can continue to control urgent urinary incontinence for six months and beyond.5 The device is not approved for stress incontinence, nor is it approved as a first-line treatment. Patients must first have failed or not responded to behavioral, medicinal and digital therapies.6
For eCoin to work, a healthcare provider implants the nickel-sized unit under the skin above the ankle, and then uses a device to program it. The healthcare provider is provided a radio to monitor the programming, and a magnet to stop the programming if need be. Patients receive a separate device to stop the implanted unit if need be.7
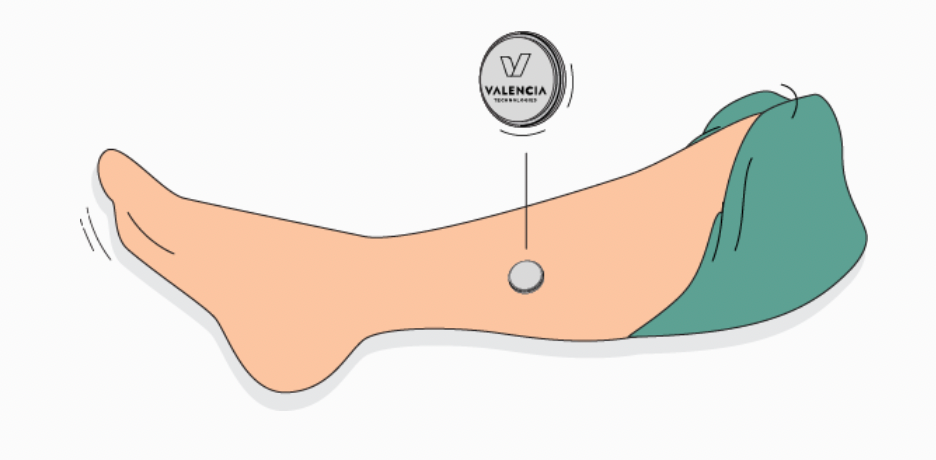
For the first 18 weeks after the implant, 30-minute stimulation sessions occur every three days, then lengthen to every four.
In a pivotal study with 133 patients, the baseline urgency episodes were at least three every day. After 48 weeks, 68% of the patients (mostly women) achieved a 50% reduction in episodes. 16% of the patients reported a device-related event by one year, post-implant.8
References
- FDA. Premarket approval. eCoin peripheral neurostimulator, March 1, 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P200036
- Cleveland Clinic. Tibial nerve. https://my.clevelandclinic.org/health/body/21962-tibial-nerve
- Patel UJ, Godecker AL, Giles DL, Brown HW. Updated Prevalence of Urinary Incontinence in Women: 2015-2018 National Population-Based Survey Data. Female Pelvic Med Reconstr Surg. 2022 Apr 1;28(4):181-187. https://pubmed.ncbi.nlm.nih.gov/35030139/
- Noridian. Posterior Tibial Nerve Stimulation Coverage https://med.noridianmedicare.com/web/jfb/policies/coverage-articles/posterior-tibial-nerve-stimulation-coverage
- Kaaki B, English S, Gilling P, et al. Six-Month Outcomes of Reimplantation of a Coin-Sized Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome With Urgency Urinary Incontinence. Female Pelvic Med Reconstr Surg. 2022 May 1;28(5):287-292.
- eCoin website. https://ecoin.us/eligibility/
- eCoin® Peripheral Neurostimulator System Patient Manual. https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200036C.pdf
- Rogers A, Bragg S, Ferrante K, Thenuwara C, Peterson DKL. Pivotal Study of Leadless Tibial Nerve Stimulation with eCoin® for Urgency Urinary Incontinence: An Open-Label, Single Arm Trial. J Urol. 2021 Aug;206(2):399-408. https://pubmed.ncbi.nlm.nih.gov/33797291/
FDA issues de novo clearance for SonoMotion’s Stone Clear device, for kidney stone treatment
Stone Clear is the first and only device cleared by the FDA that uses external ultrasound pulses to facilitate passage of post-lithotripsy kidney stone fragments without surgery.